Validation of Kv1.3 using ShK toxin
Validation of Kv1.3 channels expressed in proteoliposomes using ShK toxin
The company Synthelis, specialized in membrane protein production, has recently validated its Kv1.3 channel system using Smartox ShK toxin. Synthelis has expressed a mKv1.3 in proteoliposomes thanks to its proprietary cell-free expression system and has validated their functionality in electrophysiology. The Smartox ShK stichodactyla toxin – #08SK001 has been used to confirm the biological activity of the channel.
Kv1.3 channel background
Potassium voltage-gated channel, member 3 Kv1.3 is a voltage-gated potassium (Kv) channel that participates in cellular processes such as neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, apoptosis, cell volume regulation, and T cell stimulation. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a potassium-selective channel through which potassium ions may pass in accordance with their electrochemical gradient.
Assessment of the functionality
- Methods
The mouse potassium voltage-gated channel member 3 was expressed in Synthelis’ cell-free system in the presence of lipid vesicles. The functionality of the ion channel was evaluated by measurement of the potassium channel activity using a Port-a-Patch® instrument (Nanion Technology) in presence of the ShK known blocker toxin (from the venom of the Caribbean sea anemone Stoichactis helianthus, provided by Smartox Biotechnology).
- Results
Potassium channel activity
The figure 1 below shows the conductance at +50mV of a single mKv1.3 channel embedded into the lipid bilayer of liposomes.
 Figure 1: Single channel recording of mKv1.3 channel.
Figure 1: Single channel recording of mKv1.3 channel.
The signals were acquired after incorporation of mKv1.3 proteoliposomes into Giant Unilamellar Vesicles (GUVs) previously used to form the bilayers on the Port-a-Patch. The recordings were done at 25, 50 and 100mV (only a zoom of the recording at 50mV is shown). The recording was done at room temperature in presence of 200mM KCl.
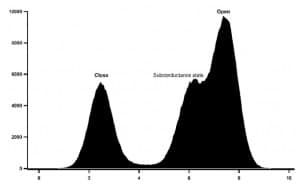 Figure 2: Histogram of mKv1.3 activity at +50mV in absence of drugs.
Figure 2: Histogram of mKv1.3 activity at +50mV in absence of drugs.
The conductance of mKv1.3 was as well calculated using all point histogram of 1800 single channel events, G=98,4 ± 6 pS at +50mV. The activity showed a voltage asymmetry of the activation, with single channel activated for voltages higher than -25mV and reaching a high open probability at +50mV. Subconductance states are also observed.
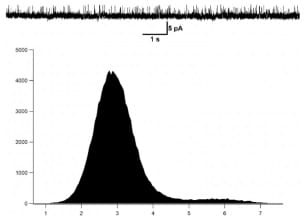 Figure 3: Histogram of mKv1.3 activity at +50mV in the presence of 100nM of the ShK toxin inhibitor.
Figure 3: Histogram of mKv1.3 activity at +50mV in the presence of 100nM of the ShK toxin inhibitor.
The conductance of mKv1.3 was as well calculated at 50mV. The application of the ShK toxin at 100nM blocks clearly the mKv1.3 activity.
Conclusion
Our data support that the cell-free protein synthesis (CFPS) in presence of appropriate liposomes, is an efficient system for the production of active ion channels suitable for patch clamp analysis. Automated patch clamp from Nanion Technology was used in this study to prove the channel activity of the mouse Kv1.3. The proteoliposome format is also compatible for manual patch clamp experiments after their incorporation into GUVs (data not shown).
View the Synthelis product’s webpage Text on the button
About Synthelis
Synthelis is a biotechnology company based in Grenoble, France, specializing in the production, purification and characterization of membrane proteins and other difficult-to-express proteins. We use a patented cell-free technology. The company is a spin-off from the University Joseph Fourier.
website: www.synthelis.com
email: contact@synthelis.fr

